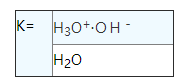The basic principle of pH measurement
Used to determine the chemical reaction process of the most familiar one of the oldest zero current measurement method is PH measurement.
What is the pH for pH measurement should know about?
Generally speaking, pH measurement is used to determine a solution of pH.
Adding acid in water, the acidity of water will increase, and the PH down. In water adding alkali, the alkalinity of water will increase, and PH value is used to indicate the PH of the unit. When we speak of milk is "cool" or acid is "weak", not sure the status of the said things, it is because we have no say measurement units and the measured values. And when we speak of milk temperature is 10 ℃, is the concept of a firm. Also when we speak of weak acid pH value of 5.2, which is a precise concept.
There are various with different acid-base strength of acid and alkali. For example: the hydrochloric acid is a kind of strong acid, and boric acid is very weak (can be used to flush the eyes and wound).
Determine the strength of the acid degree, basically see the hydrogen ions in solution of dissociation. Hydrogen ion in strong acid dissociation is very extensive, the weak acid dissociation rarely. Hydrochloric acid is a strong acid, because almost completely dissociative chlorine make hydrogen ions. Boric acid is a weak acid, because only a few hydrogen ion dissociation. Even chemical pure water has trace dissociation: strictly speaking, only before cooperate with water molecules, the hydrogen nuclei are not exist in the free state.
H2O + H2O = H3O ++ OH -
Because of hydronium ions (H3O+) concentration can be with the concentration of hydrogen ions (H+), commonly used type can be simplified into the following form:
H2O = H++ OH -
People here are hydrogen ions in a chemical is expressed as "H + ions" or "hydrogen nuclei. Hydronium nuclear is expressed as "hydronium ion. Negative hydroxyl ions called hydroxyl ions.
Using the law of mass action, can be found for pure water dissociation equilibrium constant represented:
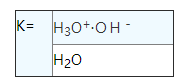
Because the water is very small amounts are dissociation, and thus the molar concentration of water in fact is a constant, and the equilibrium constant K ion product KW that ask for out of the water.
KW = K * h 2 KW = H3O +, OH - = 10 - July 10 to 7 = 10 mol/l (25 ℃)
That is to say, for a litre of pure water at 25 ℃ is 10-7 10-7 Moore Moore H3O ions and OH - ions.
In order to avoid operations are carried out with this negative ghost molar concentration index, biologists zebulon sen (Soernsen) in 1909 suggested that the inconvenience to use numerical replace with logarithmic, and defined as "pH". Mathematics defined on the pH value of hydrogen ion concentration of the logarithm of a negative value. That is:
PH = logH +
Strictly speaking, this formula ignores the hydrogen ions (H +) and hydroxyl ions (OH -) interaction, because between ions, the effect of electric field force makes the ion activity significantly decreased. That is to say: the concentration of hydrogen ions play a role (activity) is related to dissolve all the other ions.
For example: when the concentration of hydrogen ions for 10-1 mol / 1, the pH value should be 1.0 in theory, but we only measured pH value of 1.08. This means that degree coefficient indicates 1 f, but 0.823. That is to say, should be an exact definition of pH: pH
Temperature coefficient of the measuring solution:
Due to the ion product are very much dependent on the temperature, water distribution of the neutral point has the following:
0 ℃ = pH
25 ℃ = pH
75 ℃ = pH
100 ℃ = pH
Acid and alkali is diluted with water, is sure to have the pH dependent on temperature.
For strong acid, water dissociation is zero, the influence of pH value is only determined by the acid dissociation:
0 ℃ 25 ℃ 50 ℃
0.001 nHCL 3.00pH 3.00pH 3.00pH
0.1 n HCL 1.08pH 1.08pH 1.08pH
In alkaline solution, the effect is very big. Because the hydrogen ion activity decreased, and at the same time water dissociation of self advantage.
0 ℃ 25 ℃ 50 ℃
0.001nNaOH 11.94pH 11pH 10.26pH
Saturated limewater ┄ ┄ pH 11.68, pH 12.4
For practical, have the following conclusion:
For the process control of PH value, must also know the temperature of the solution properties; Only under the condition of the measured in the same temperature to its PH value in the comparison.
How to make the PH measurement?
Almost everyone knows that using litmus paper or change with PH value of the different characteristics of color measurement method. Such as litmus paper into a dark red or light red in the acid solution, while in alkali solution into the deep blue or light blue.
But this method in weak buffer, or organic compound containing metal ions in the solution or there will be a significant error in the solution (ph 2 or less).
In order to get accurate and repeatable pH value, potential analysis method are used to pH measurement.