Redox measurement and the scale of the pH value is proton H+ activity. Redox voltage gives the electronic activity. In the pH and Redox reaction activity of voltage is measure is intended to show, the difference only lies in the display scale is a pH, and the other is the mv.
For two kinds of measurement, the electrical potential difference of voltage measurements made by zero current. Although the Redox sensitive precious metal electrode is used in the measurement of low resistance electrical conductor, Redox voltage still need to measure high impedance amplifier.
The other 2 in common and electrode technology, and the changes of the measured potential follow nernst equation.
The implications of REDOX or (ORP)
These two abbreviations is very good translation. REDOX Reduction and Xidation two words of prefixes, and ORP is Oxidation Reduction Potential of the three words of morpheme.
For Redox measuring its basic principle, for the user is more or less is not clear.
Such as hydrochloric acid (HCl) containing H + protons, and there is a positive unit charge, is should be instead of negative ions, here is the Cl -. Negative charge carrier is electron.
When we put zinc and iron in hydrochloric acid, will generate hydrogen, which can turn iron or iron oxide. Molecular transformation has happened here. Combined with Cl - hydrogen reduction of H+ ions. And the hydrochloric acid and iron what happens?
Each element and each compound has its own electrochemical potential (relative standard hydrogen electrode potential). The negative matter potential/high polarity will electronically transmitted to potential/low polarity is positive material. Accept electronic materials will be, that is to say from the ionic form, convert () for hydrogen gas form or form (for metal ions) pure metal. Lost electronic forms of material will be converted into ions.
In our case,
Hydrogen (H+) potential is 0.0 V (free) in hydrochloric acid, iron (Fe) potential is 0.4 V
Iron is given two electrons and form into ions. Hydrogen ions accept the two electrons and converted to hydrogen - in the form of hydrogen gas.
The above process through reactive may see more clearly.
Fe =Fe2+ + 2 e
2 e+2H+ = H2
-- -- -- -- -- -- -- -- --
Fe + 2 H + = Fe2+ + H2
Accept electronic material reduction, that is to say, from oxidation state into a square one. Lose the electronic materials are oxidation, that is, from the square one also transformed into oxidation state. Here is another example:
Zinc potential is 0.76 V, the potential of copper is + 0.337 V.
Insert the zinc in copper sulfate solution, need not impressed current has copper precipitation on the zinc bar.
(0.76 V) Zn = Zn2+ + 2 e
2 e + Cu2 + = Cu (+ 0.337 V)
-- -- -- -- -- -- -- -- -- -- -- -- -- -- --
Zn + Cu2 += Zn2+ + Cu
Zn is given two electrons to copper ions, copper and copper ions reduction into metal. If there is a potential difference, will be from high potential to low potential to create a current.
Potential difference is the electromotive force (EMF).
To illustrate this phenomenon, we suppose there are two POTS, one is full of water. The lower pipe connecting the two POTS. When the valve is opened, the water will flow from POTS full of water to the empty jar. From this kind of phenomenon we can see, there is no oxidation reduction process, nor without the process of oxidation reduction process.
Oxidation process system general voltage in the range of + 3 v to 3 v. The higher the potential difference, has high potential to low potential of reducing or from low potential to the stronger the oxidation of high potential. Oxidizing and reducing strength is not absolute, but is related to the following factors:
A) reference medium concentration
B) the size of the potential difference
C) the pH
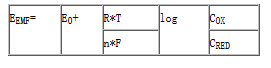
From the nernst equation can clearly see that the concentration of "C", the number of electronic "n" and pH "E0" is how to play a role.